Recommended DePuy Hip Attorneys in New York - Trusted lawyers in your area
Hip replacement, also referred to as hip arthroplasty, is a surgical procedure where a damaged or diseased hip joint is replaced with an artificial joint or prosthesis. Two medical devices used in hip implant procedures — the DePuy ASR™ XL Acetabular Hip System and DePuy ASR™ Hip Resurfacing System — may have a higher fail rate than other hip replacement devices and could cause patients to experience significant complications.
If you had a hip replacement procedure after 2003 with a device made by DePuy Orthopaedics, you should consult with your physician for an evaluation to determine if surgery or other corrective care is required. You should also speak with respected product liability attorneys from Belluck & Fox to find out if you have any legal recourse for defective metal-on-metal hip implants.
Product Information – DePuy Hip Lawsuit
Table of Contents
- 1 Product Information – DePuy Hip Lawsuit
- 2 Problems With DePuy Hip Implants
- 3 DePuy Hip Implant Complications
- 4 Other Side Effects Related To DePuy Hip Replacements
- 5 Damage From DePuy Hip Replacement Products
- 6 Metal-on-Metal Hip Replacements Have Higher Rate of Complications
- 7 FDA Seeking Expert Advice on Risks of Metal-on-Metal Artificial Hips
- 8 Get Help From Our Defective Hip Implant Lawyers Today
Your hip joint is referred to as a ball and socket joint. Patients with damaged or diseased hip joints may undergo either a total hip replacement or a hip resurfacing operation that replaces or resurfaces this joint.
When you undergo a hip joint replacement, a metal cap is fitted onto the femoral head while the socket is lined with an artificial cup.The cup may be held in place by affixing screws to the pelvis, or a special material may be used that promotes regenerative bone growth and causes the bone to grow onto the femoral stem and acetabular cup to anchor the components in place. The metal head fits into the artificial cup.
In a total hip replacement, on the other hand, the top of your femur is actually removed and the stem of the femur is hollowed out. A metal stem is then fit into the carved-out femur and a new artificial metal head is placed onto the stem. A cup is also placed into the socket. Essentially, this creates a whole new ball and socket joint.
There are many different products used for the femoral stem and the artificial cup. For a long time, polyethylene-lined replacement cups, along with ceramic or metal femoral heads, were used. Unfortunately, the polyethylene liner was prone to wearing out and needing replacement, especially in active people, while the ceramic hip components were prone to breakage or chipping in active individuals. Some patients also reacted when surface wear caused polyethylene particles to accumulate in the body.
DePuy hip implants provided an alternative by offering metal-on-metal components. Although available since the 1960’s, metal-on-metal hip replacement components were not widely used until recently. Their resurgence was driven in part by the development of high-tech hard metal alloys and processes that allow for the metal components to fit perfectly together. Metal-on-metal hip replacements were especially common for patients who were younger and more active. A 2011 article published in the British Medical Journal indicated that metal-on-metal was used in 10 percent of total hip operations between 2006 and 2009.
DePuy Orthopaedics was one company that made metal-on-metal hip products and had two widely used hip replacement systems. One, the DePuy ASR™ XL Acetabular System, was used for total hip replacement procedures. The other was the DePuy ASR™ Hip Resurfacing System, which was never approved for use in the United States.
An estimated 93,000 patients worldwide had one of the two DePuy hip replacement systems implanted. Despite its lack of U.S. approval, many Americans had the DePuy ASR™ Hip Resurfacing System implanted either abroad or as part of U.S. clinical trials, according to a statement a DuPuy spokesman made to Arthritis Today.
Unfortunately, while the tight precise fit and the smooth surface of the metal led manufacturers to promote the pharmaceutical products as a long-lasting alternative, problems soon began to develop.
Problems With DePuy Hip Implants
Many of the patients who underwent hip implant surgery with hip replacement products from DePuy Orthopaedics are now facing medical problems, leading them to file lawsuits against DePuy for compensation.
Some examples of problems that can result include:
- Aseptic loosening: The components used in the hip replacement come loose. This type of loosening is not caused by infection or disease and most often involves the acetabular cup. Once loosening occurs, surgical correction is the only solution.
- Osteolysis: This refers to bone loss that occurs as a result of the release of metal ions into the body. Because of this bone loss, the bony growth holding some hip replacement devices in place is inhibited or reversed, which could be a potential cause of loosening. Again, a surgical procedure is normally necessary to correct the problem.
- Metallosis: Metallosis refers to an adverse reaction to the build-up of metal ions or debris from the metal hip replacement components. Complications may include tissue death; bone degradation; and the development of a collection of metal stained fluid known as a psuedotumor. Removal of the metal ions and debris is the only treatment.
- Neurological problems. Long-term exposure to cobalt and chromium ions may cause a number of neurological problems with symptoms including headaches; a metallic taste in the mouth that doesn’t go away; memory problems; and heart abnormalities.
DePuy Hip Implant Complications
Many of the patients who underwent hip implant surgery with hip replacement products from DePuy Orthopaedics are now facing new medical problems.
News of the possible complications comes directly from DePuy itself. The company voluntarily recalled both the ASR™ XL Acetabular Hip System and the DePuy ASR™ Hip Resurfacing System in the United States in August 2010. The New York Times reported that the U.S. Food and Drug Administration (FDA) had received complaints about DePuy hip implant failures as early as 2008.
The recalls occurred after unpublished data from the United Kingdom’s joint registry revealed a higher failure rate for DePuy devices than other hip replacement devices. According to Arthritis Today, the data leading to the recall showed that around 12 percent of patients with a DePuy hip implant would need a corrective procedure within just five years. Kevin J. Bozic, MD, an orthopedic surgeon and vice chairman of orthopedics at the University of California at San Francisco Medical Center, told Arthritis Today that this fail rate was about twice the industry average.
Data presented at the British Hip Society Annual conference in March 2011 further confirmed the high fail rate of DePuy devices. The data indicated that 49 percent of people with a DePuy device would need to have the device replaced within six years. According to a January 2012 U.S. Library of Medicine article, this number is considerably higher than other metal-on-metal devices that have a five-year replacement rate of between 12 and 15 percent.
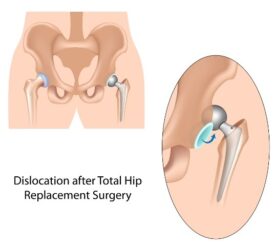 Tiny metal particles that can flake off of hip replacements are another concern. The FDA issued a statement about the potential for metal-on-metal hip replacements like DePuy’s to shed metal ions. Those particles could cause damage to the bone, muscle and other tissue around the hip and the implant, according to the FDA. The FDA statement also said that “there are some case reports in the literature of a small number of patients in which high levels of metal ions in the bloodstream may have caused other types of symptoms or illnesses elsewhere in the body, including effects on the heart, nervous system, and thyroid gland.”
Tiny metal particles that can flake off of hip replacements are another concern. The FDA issued a statement about the potential for metal-on-metal hip replacements like DePuy’s to shed metal ions. Those particles could cause damage to the bone, muscle and other tissue around the hip and the implant, according to the FDA. The FDA statement also said that “there are some case reports in the literature of a small number of patients in which high levels of metal ions in the bloodstream may have caused other types of symptoms or illnesses elsewhere in the body, including effects on the heart, nervous system, and thyroid gland.”
An investigation by the British Medical Journal (BMJ) and BBC NewsNight in February 2012 reported that the release of ions from the metal-on-metal implants could cause long-term disability, as well as destroy muscle and bone. The ions can also spread to the lymph nodes, spleen, liver and kidneys through the bloodstream before being excreted in urine, according to the report.
The BMJ and NewsNight investigation also brought attention to an internal DePuy memo that allegedly shows DePuy knew there was a risk of immune function change from the release of ions. The memo also reportedly discussed a possible concern that the debris from the breakdown of the metal joint might be carcinogenic. These concerns were based, in part, on past studies indicating that patients with a joint replacement had a three times greater chance of developing lymphoma and leukemia. The U.S. National Library of Medicine, however, indicates that there is not yet conclusive proof that metal in the bloodstream from hip implant devices harms the body beyond damaging the soft tissue near the implant.
Damage From DePuy Hip Replacement Products
Patients whose DePuy hip replacement devices fail prematurely may need additional corrective surgery. Those with ASR hip replacement parts may also experience discomfort, infection to surrounding tissue and difficulty walking. Patents should be compensated not only for the cost of medical monitoring and additional surgeries related to defective hip replacement parts, but also for the pain and suffering they had to endure.
Metal-on-Metal Hip Replacements Have Higher Rate of Complications
People who have deterioration or damage to their hip joints caused by rheumatoid arthritis, hip fractures or insufficient blood supply may undergo hip resurfacing or total hip replacement surgery which is called hip arthroplasty. A ball at the end of the thigh bone fits in a socket in the pelvis, forming the hip joint.
Metal-on-metal artificial hips, in which the ball and socket are both made of metal, have become popular for hip replacement procedures in recent years.
The two categories of hip replacements are:
- Metal-on-metal total hip replacement, consisting of a metal ball (femoral head), a metal stem attached to the thigh bone and a metal cup in the hip bone;
- Metal-on-metal hip resurfacing system, consisting of a trimmed thigh bone capped with a metal covering and a metal cup in the hip bone.
Manufacturers have marketed the metal-on-metal artificial hips as lasting longer and being well suited for younger patients who wish to remain physically active.
But metal-on-metal hips appear to have a higher failure rate and require follow-up surgeries more often than other types of artificial hips, based on data from national registries that track hip replacement patients in the United Kingdom and Australia.
A recent study based on data from the United Kingdom registry noted an increased failure rate involving total hip replacements that use large-diameter metal balls, which are known as femoral heads. Larger metal balls can generally cause more wear.
FDA Seeking Expert Advice on Risks of Metal-on-Metal Artificial Hips
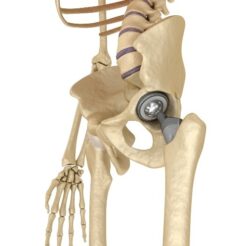 The U.S. Food and Drug Administration, which regulates medical devices, is considering whether to require more rigorous testing of artificial hips before allowing them on the market because of reported complications with the devices.
The U.S. Food and Drug Administration, which regulates medical devices, is considering whether to require more rigorous testing of artificial hips before allowing them on the market because of reported complications with the devices.
The FDA said in March 2012 that the recent study of higher failure rates involving some artificial hip systems has added to the agency’s existing concerns about the safety of metal-on-metal (MoM) hip systems.
A common reason for metal-on-metal hip failure is loosening of the components.
As the metal-on-metal surfaces of the artificial hips wear, they may flake off metal particles. The release of metal ions into the blood stream can be detrimental to the bone growth needed to hold the artificial joints in place. As a result, hip replacements that seem to work well immediately after surgery become loose over a period of months as bone deteriorates.
In 2011, the FDA ordered 21 medical device manufacturers to do post-market studies to collect more safety data regarding problems involving metal-on-metal hips. Some patients who have received metal-on-metal artificial hips have reported headaches, a metallic taste in the mouth, memory problems and heart problems. The FDA said the studies will help the agency to gain a better understanding of the safety of the devices.
The United Kingdom’s Medicines and Healthcare products regulator agency in 2010 issued a safety alert advising patients with painful metal-on-metal hip replacements to have MRIs and blood tests to see if metal particles from the artificial hips were flaking off and getting into their bloodstream. The body’s reaction to metal debris in the body may cause a condition known as metallosis. This can lead to various complications, including death of tissue and damage to bones.
Until recently, the United States has not had a national registry similar to the UK registry to track complications with medical devices used in hip replacements. The American Academy of Orthopaedic Surgeons is engaged in establishing a national joint registry. The registry will help doctors identify patterns of problems with malfunctioning artificial hips.
Get Help From Our Defective Hip Implant Lawyers Today
Belluck & Fox, LLP has more than a decade of experience representing clients injured by faulty products and defective medical devices. Our experienced NYC personal injury attorneys can help patients fight for their right to be fairly compensated for the discomfort and inconvenience caused by defective medical devices.
Contact our product liability lawyers at Belluck & Fox, LLP immediately for a free legal evaluation of your defective hip implant claim. Call our NYC law office today, (212) 681-1575 or contact us via our online form.